

Structure determination and domain interaction of the photochromic protein YtvA from Bacilus subtilis |
 |
 |
|
 |
||
|
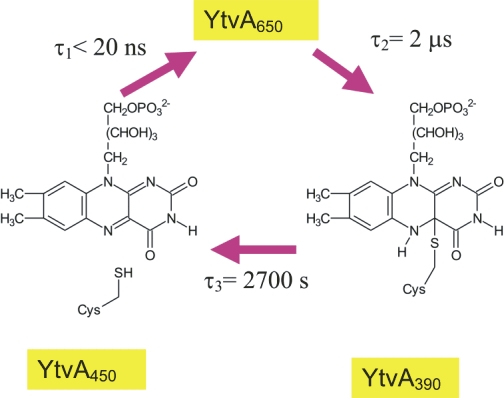
Light is an important stimulus for plants and various bacteria. These organisms have therefore developed a number of photoreceptors, usually consisting of a protein and an organic cofactor, to sense light of different wavelengths. The detailed mechanism of light perception, i.e., the "primary event" and the consecutively following conformational changes of the receptor, are still only poorly understood. In this project the structure and domain interaction of the flavin-binding, blue-light photoceptor YtvA from Bacillus subtilis, that is part of the stressosomal complex will be studied. This photoreceptor is a two-domain protein, consisting of a LOV (light, oxygen, voltage) domain (harboring a flavin chromophore) and a STAS (Sulphate Transporters AntiSigma-factor antagonist) domain. Utilizing modern NMR-techniques in conjunction with isotope-labeling, we have determined the three-dimensional structure of YtvA, studied a variety of biophysical properties of the protein and its domains and have attempted to propose a mechanism for the signal transduction within the stressosome. |
|
Publications |
||
| M. Jurk; P. Schramm; P. Schmieder*; "The blue-light receptor YtvA from Bacillus subtilis is permanently incorporated into the stressosome independent of the illumination state"; Biochem. Biophys. Res. Commun. 00, 000-000 (2013) |
| M. Dorn; M. Jurk; P. Schmieder*; "Blue News Update: BODIPY-GTP Binds to the Blue-Light Receptor YtvA While GTP Does Not"; PLoS ONE 7, e29201 (2012) |
| M. Jurk; M. Dorn; P. Schmieder*; "Blue Flickers of Hope: Secondary Structure, Dynamics and Putative Dimerisation Interface of the Blue-Light Receptor YtvA from Bacillus subtilis"; Biochemistry 50, 8163-8171 (2011) |
| M. Jurk; M. Dorn; A. Kikhney; D. Svergun; W. Gärtner; P. Schmieder*; "The switch that does not flip - the blue-light receptor YtvA from Bacillus subtilis adopts an elongated dimer conformation independent of the activation state as revealed by a combined AUC and SAXS study"; J. Mol. Biol. 403, 78-87 (2010) |
last changes 01.03.2013, Peter Schmieder