| Structure of the chromophore in phytochrome from Synechocystis sp. PCC6803 |
 |
 |
|
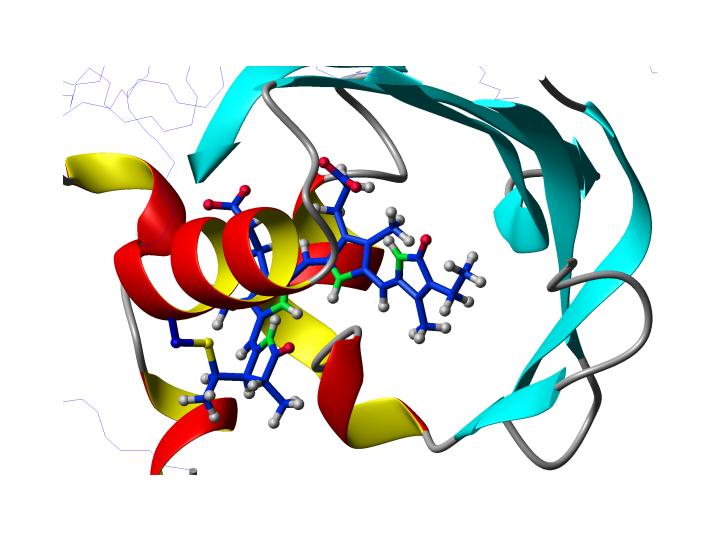
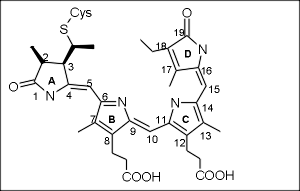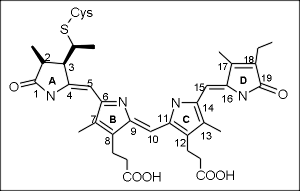
Goal of this part of the project is the determination of a structure of the chromophore in two different prokaryotic phytochrome-type proteins using solution state NMR spectroscopy. The two proteins are Cph1 from Synecchocystis sp. PCC6803 and Agp1 from Agrobacterium tumefaciens, that both bind linear, open-chain tetrapyrrole chromophores. Using appropriately labeled samples structural information about distances and angles will be extracted from Nuclear Overhauser effects (NOEs) and residual dipolar couplings (RDCs), respectively. We will use fully deuterated protein in conjunction with either unlabeled, 15N labeled or uniformly 13C labeled chromophore. Differences between the Pr and Pfr state with respect to structure will be elucidated. |
|
Publications |
||
| Y. Yang; M. Linke; T. von Haimberger; J. Hahn; R.A. Matute; L.González; P. Schmieder; K. Heyne* ; "Real-time tracking of phytochrome's orientational changes during Pr photoisomerization"; J. Am. Chem. Soc. 134, 1408-1411 (2012) |
| M. Röben; J. Hahn; E. Klein; T. Lamparter; G. Psakis; J. Hughes; P. Schmieder*; "NMR Spectroscopic Investigation of Mobility and Hydrogen Bonding of the Chromophore in the Binding Pocket of Phytochrome Proteins"; ChemPhysChem. 11, 1248-1257 (2010) |
| M. Mroginski*; D. von Stetten; F. Escobar; H. Strauss; S. Kaminski; P. Scheerer; M. Günther; D. Murgida; P. Schmieder; C. Bongards; W. Gärtner; J. Mailliet; J. Hughes; L.-O. Essen; Peter Hildebrandt*; "Chromophore Structure of Cyanobacterial Phytochrome Cph1 in the Pr State: Reconciling Structural and Spectroscopic Data by QM/MM Calculations "; Biophysical J. 96, 4153-4163 (2009) |
| J. Hahn; H.M. Strauss; P. Schmieder*; "Heteronuclear NMR Investigation on the Structure and Dynamics of the Chromophore Binding Pocket of the Cyanobacterial Phytochrome Cph1"; J. Am. Chem. Soc. 130, 11170-11178 (2008) |
| J. Hahn; R. Kühne; P. Schmieder*; "15N solution-state NMR study of a-C-phycocyanin. Implications for the structure of the chromophore binding pocket of the cyanobacterial phytochrome Cph1 "; ChemBioChem 8, 2249-2255 (2007) |
| T. Rohmer; H. Strauss; J. Hughes; H. de Groot; W. Gärtner; P. Schmieder; J. Matysik*; "15N MAS NMR studies of Cph1 phytochrome: chromophore dynamics and intramolecular signal transduction"; J. Chem. Phys. B 110, 20580-20585 (2006) |
| J. Hahn*; H. Strauss; F. Landgraf; H. Faus Gimenèz; G. Lochnit; P. Schmieder; J. Hughes; "Probing protein-chromophore interactions in Cph1 phytochrome via mutagenesis"; FEBS J. 273, 1415-1429 (2006) |
| H.M. Strauss*; P. Schmieder; J. Hughes; "Light-dependent dimerisation in the N-terminal sensory module of cyanobacterial phytochrome 1 (Cph1D2)"; FEBS Lett. 579, 3970-3974 (2005) |
| H.M. Strauss; J. Hughes; P. Schmieder*; "Heteronuclear solution-state NMR studies of the chromophore in cyanobacterial phytochrome Cph1"; Biochemistry 44, 8244-8250 (2005) |
last changes 01.03.2012, Peter Schmieder